-
Nu.Q® Technology
Our technology detects characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer, sepsis and other diseases.
-
- Our Tests
- Human Health
-
Nu.Q® NETs
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis.
-
Nu.Q® Discover
Buy our Nu.Q® Discover H3.1 Research Use Only Assay
- Animal Health
-
Nu.Q® Vet Cancer Test
Nu.Q® Vet Cancer Test detects 76% of systemic cancers at 97% specificity.
An epigenetic solution to help detect, guide treatment, and monitor cancer.
Nu.Q® Cancer
Cancer
Cancer is a devastating disease that touches many peoples’ lives, accounting for approximately 10 million deaths worldwide each year.
Overview
Cancer is the second leading cause of death globally and exerts an enormous burden on families, communities, and health systems. Survival rates are improving in countries with strong health systems, thanks to advances in cancer detection and treatment. However, access to timely diagnostics and therapies remains limited for cancer patients in low and middle-income countries.
Cancer cases worldwide
Lung Cancer cases
Ovarian Cancer cases
Non-Hodgkin Lymphoma cases
Our Solution
We are developing Nu.Q® Cancer – a range of simple, cost-effective blood-based assays.
Our patented Nucleosomics™ technology isolates circulating nucleosomes from the blood for quantification and analysis.
Nu.Q® can detect characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer and has potential applications beyond cancer detection. Being able to use epigenetic information from the nucleosomes of tumor cells could help physicians:
- Predict treatment response for each patient.
- Monitor treatment response and disease progression.
- Amend a patient’s cancer treatment regimen as quickly as possible.
Nu.Q® Cancer could also play a pivotal role in Minimal Residual Disease monitoring.
Nu.Q® Cancer
Focus areas.
We are currently investigating the potential use of Nu.Q® tests in a range of cancers and clinical settings.
Disease and treatment monitoring
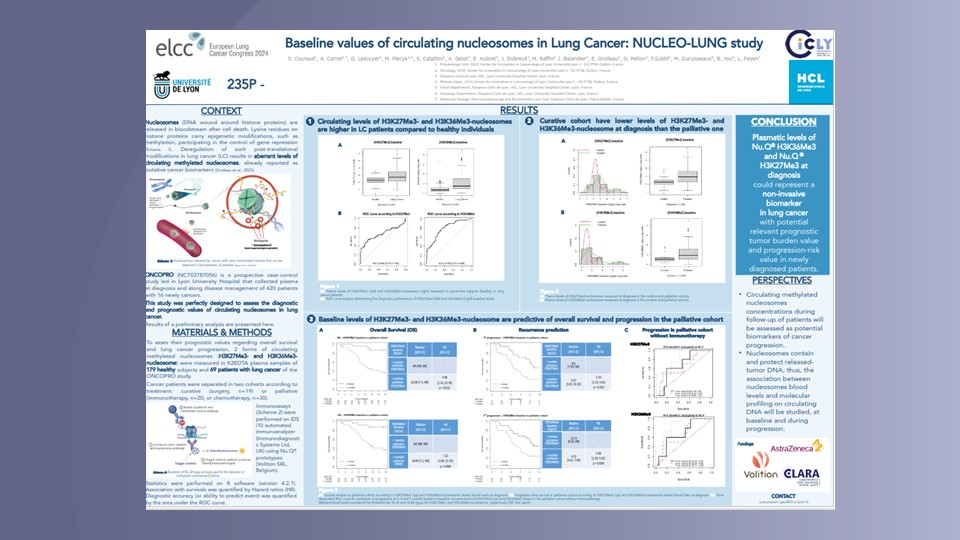
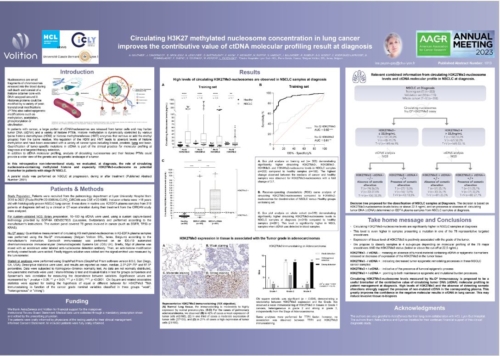
Nu.Q® Cancer can be used at diagnosis to measure and monitor nucleosome levels and modifications in circulating blood. It provides a baseline for oncologists and may enable them to predict how effective a treatment will be for a patient. They could also use our test throughout treatment to track a patient’s disease progression – potentially making timely, vital changes to their treatment regimen to ensure the best outcome.
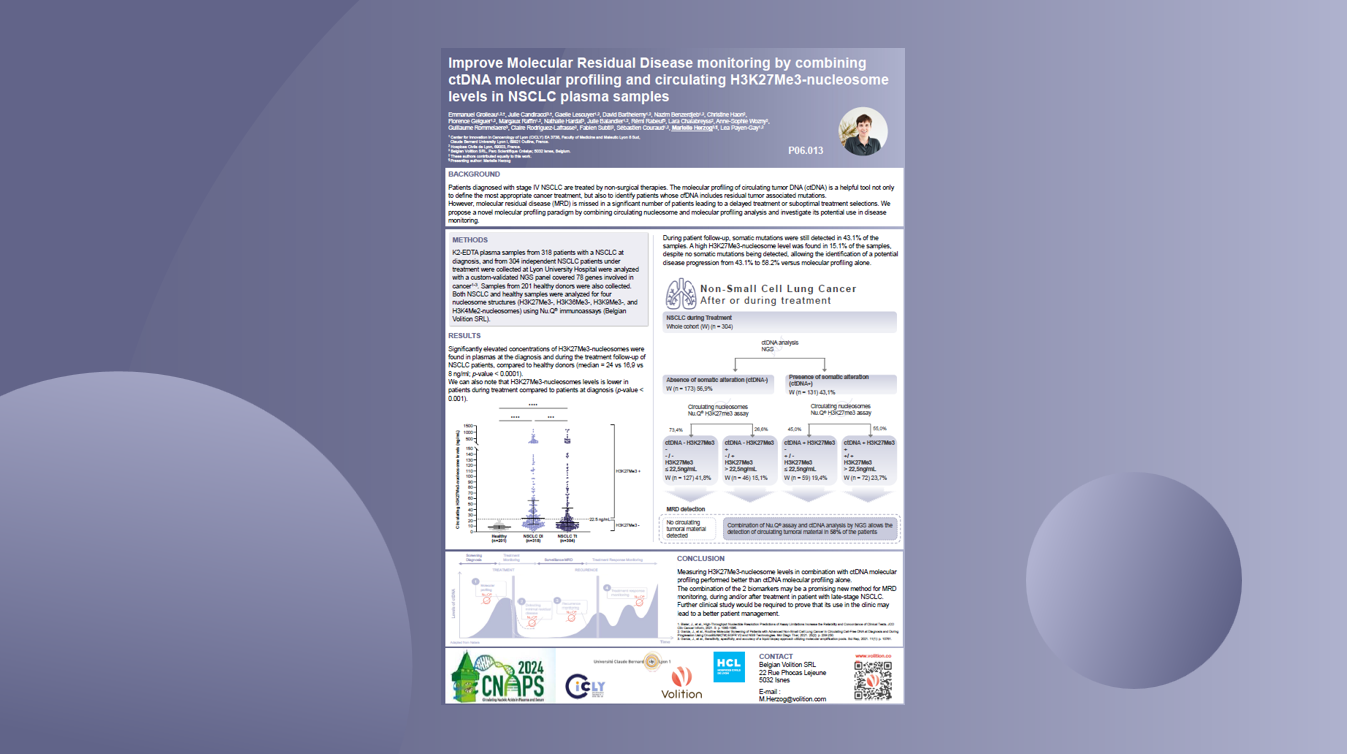
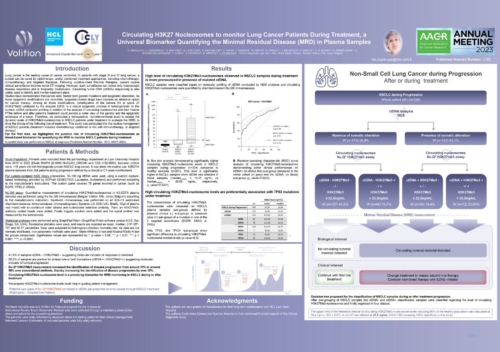
Minimal Residual Disease Monitoring
Nu.Q® Cancer could also play a pivotal role in Minimal Residual Disease Monitoring – to quantify and monitor the small number of cancer cells left in the body after cancer treatment, which have the potential to cause relapse.
Our simple, routine blood test could help catch signs of the disease coming back early, guide decision-making and an oncologist’s next steps.
Collaboration
Working together.
We are working with teams at clinical centers of excellence worldwide, as they undertake studies to test our technology as an aid for cancer detection, disease progression and treatment response monitoring.
Find out more
Download our brochure for more details about the potential predictive, diagnostic, and prognostic value of Nu.Q® Cancer in clinical settings.

Product Resources
Brochure & Clinical Papers
Contact Nu.Q® Cancer Team
Modal Contact Form (Cancer)
"*" indicates required fields